The EU's New Clinical Trial Regulation: How Sites and Sponsors Can Prepare for the Change | CenterWatch
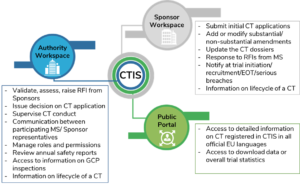
Adapting to the Evolving European Clinical Trial Regulatory Scenario: An Overview of the Current State of the European Clinical Trials Regulation and Clinical Trials Information System - ACRP

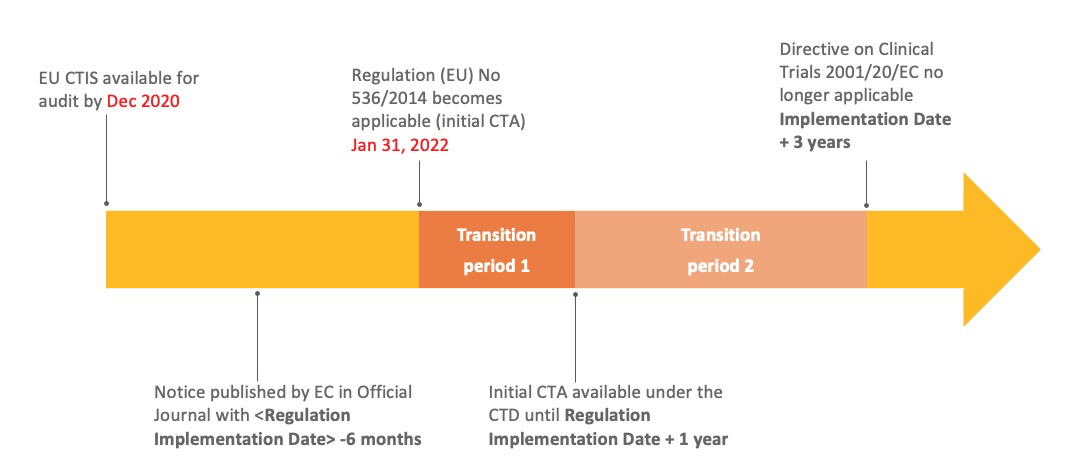
Key considerations to be aware of with Clinical Trial Regulation (CTR) EU No 536/2014 | LINK Medical