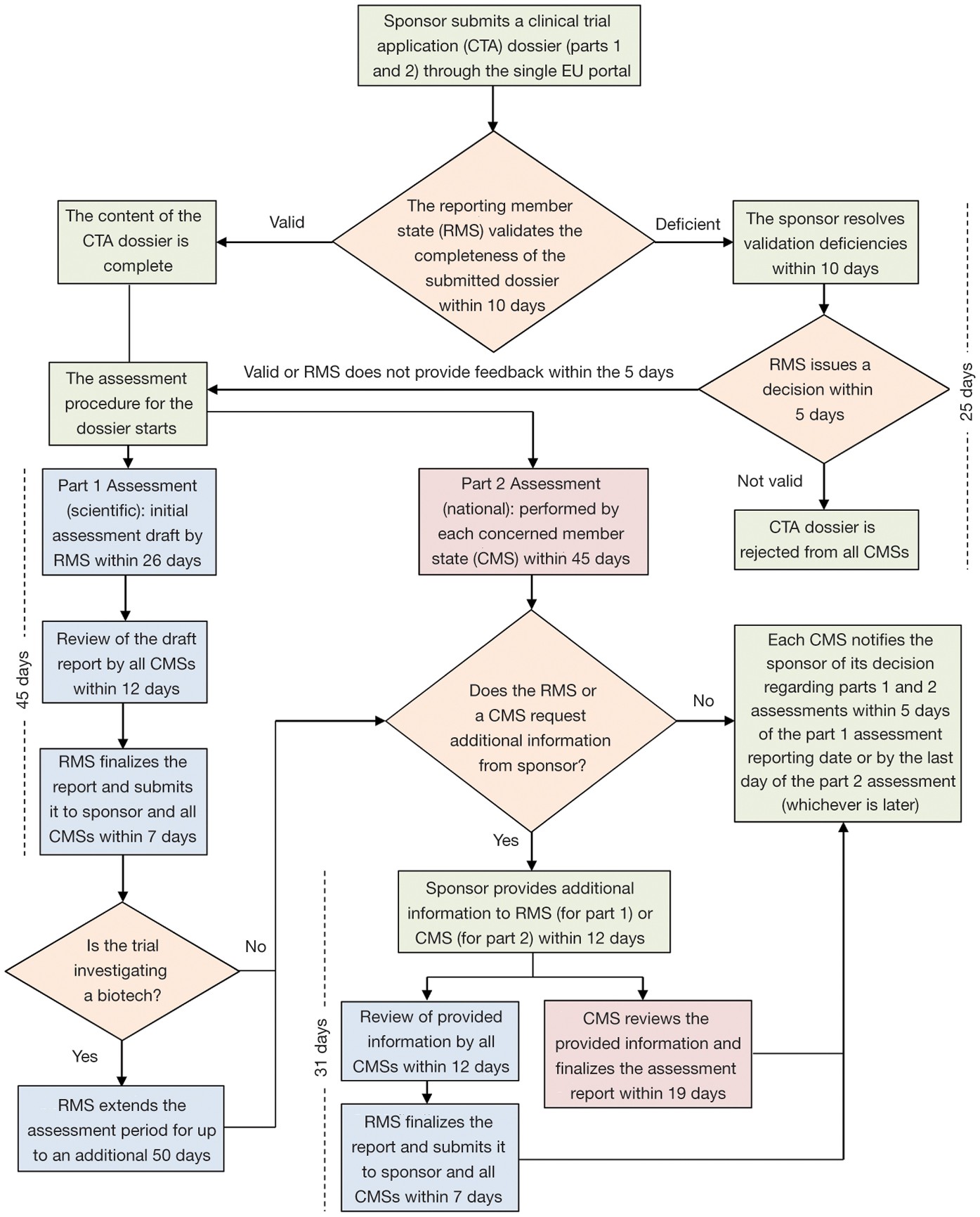
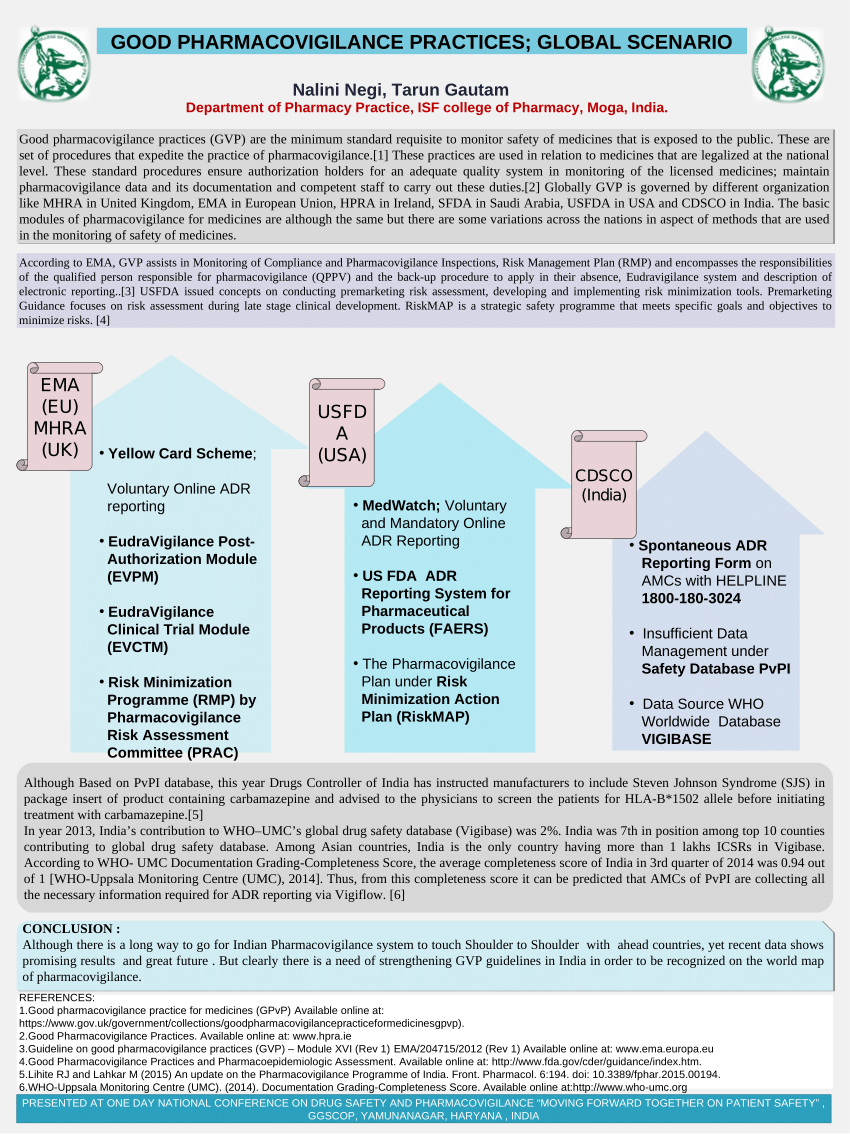
Adverse event (AE) reporting algorithm. Timeframe for adverse event... | Download Scientific Diagram

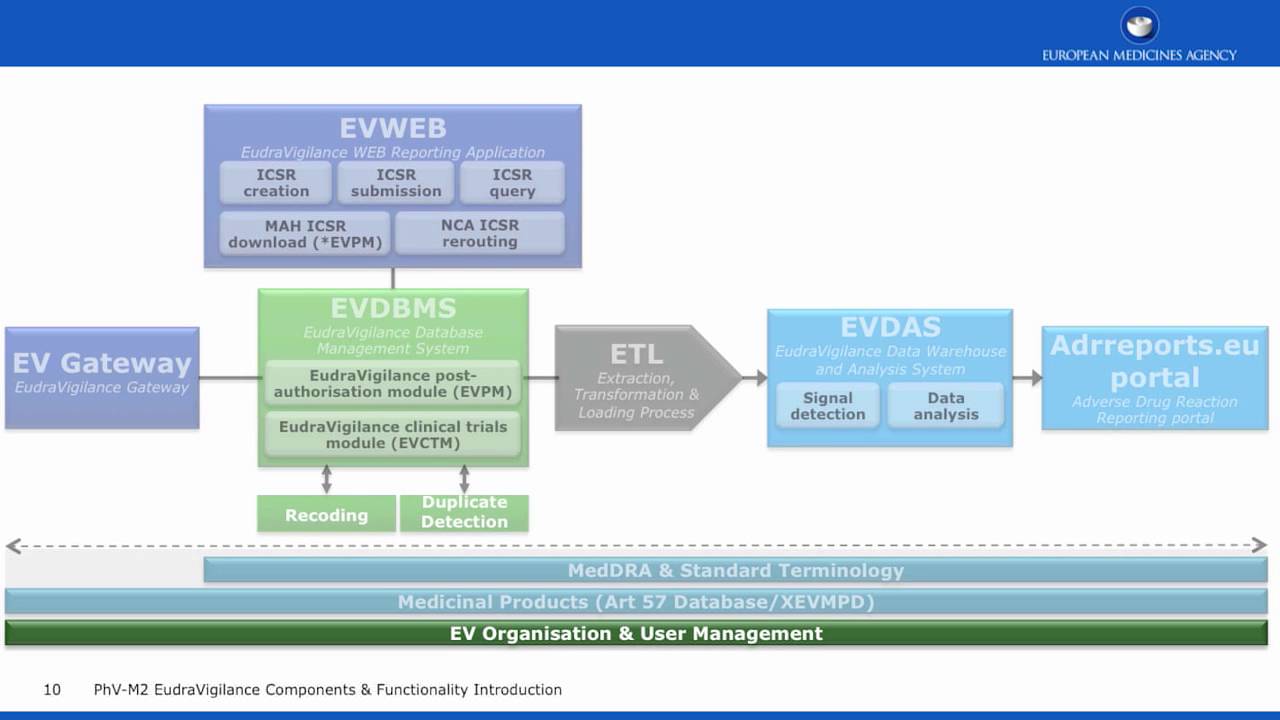
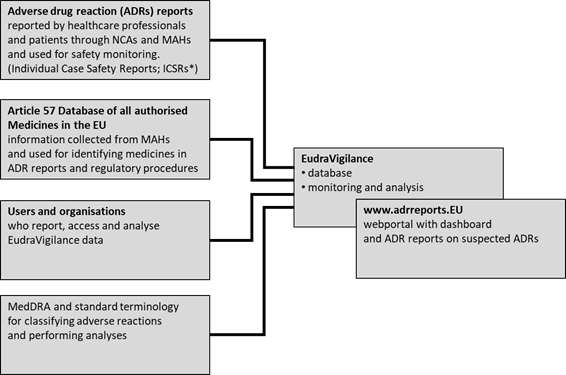
Dr. Shripadaraja.R on Twitter: "EudraVigilance system: Each component explained in detail: Part 4 MedDRA: #drugsafety #pharmacovigilance #clinicaltrials #lifesciences #healthcare #regulatory #medicine #research # clinical #ema #fda #pharma #biotech ...
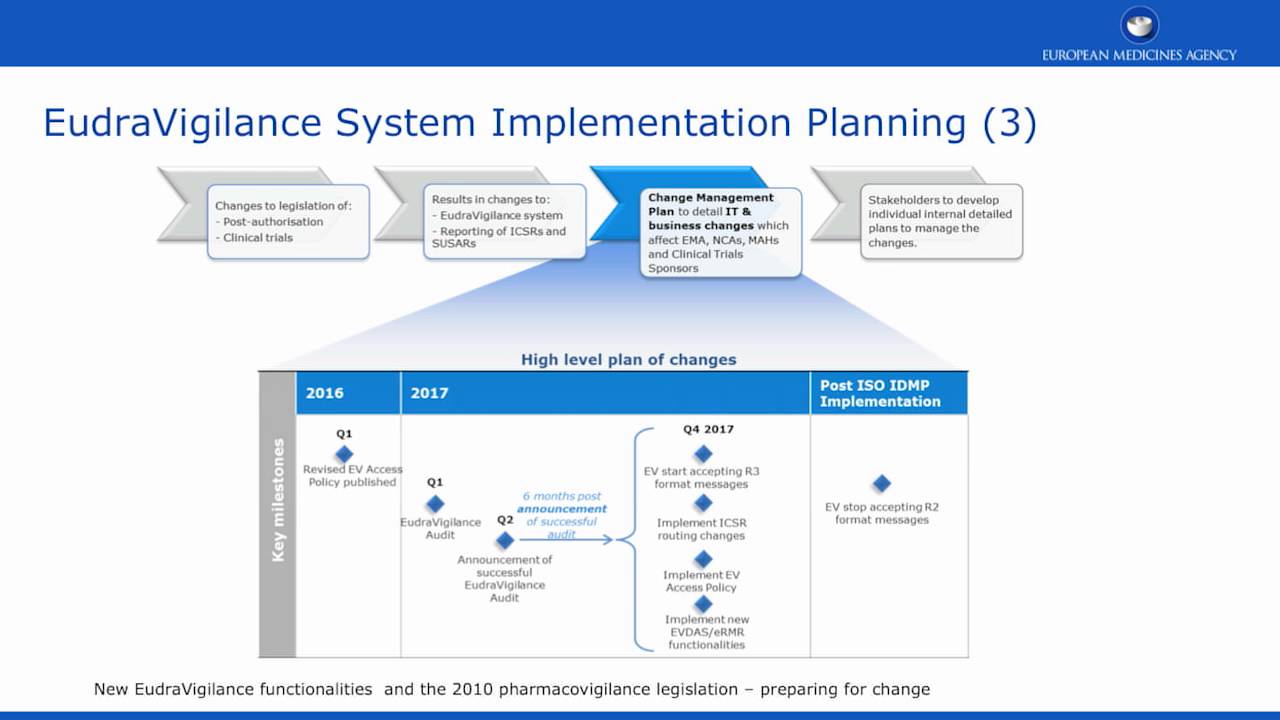
London, 01 February 2008 Doc. Ref. EMEA/553390/2007 NOTE FOR GUIDANCE EUDRAVIGILANCE HUMAN VERSION 7.1 PROCESSING OF SAFETY MESS
Phone: +40-21 .31 7.11 .02 Fax: +40-21.316.34.97 Electronic Reporting of Suspected Unexpected Serious Adverse Reactions (SUSARs)