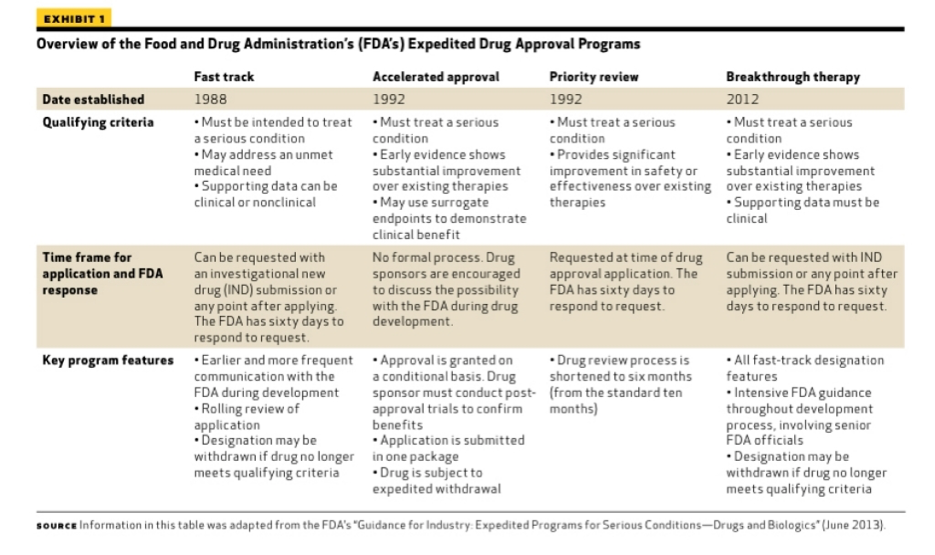
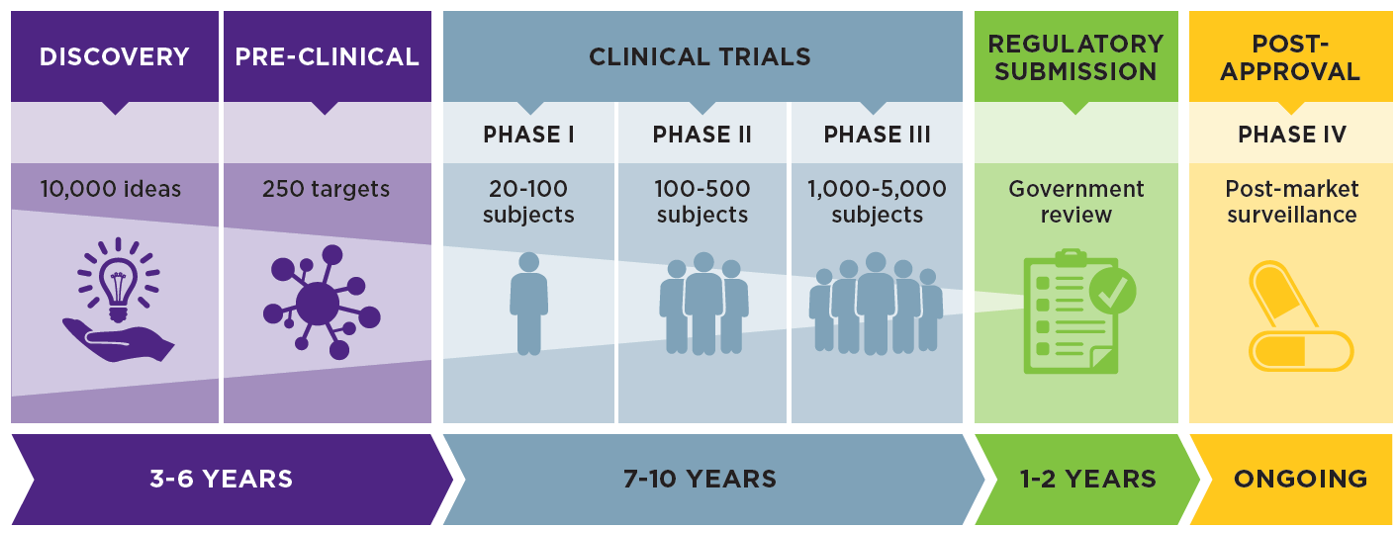
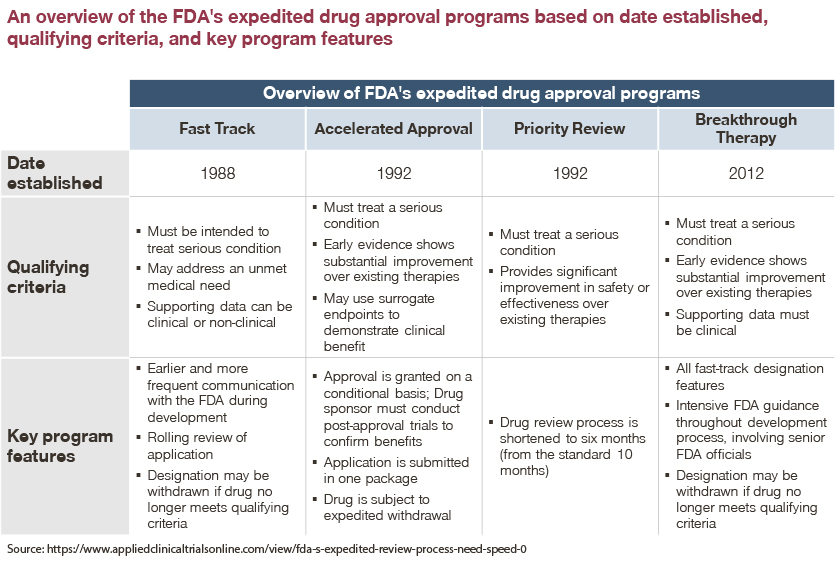
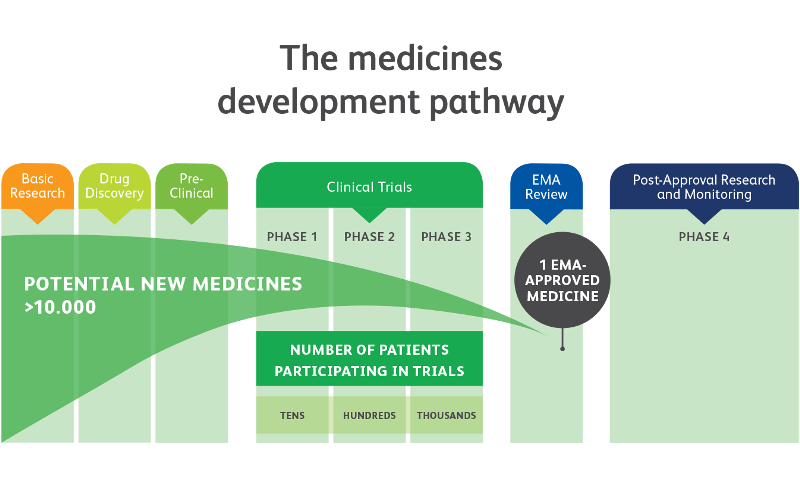
Effects of an Integrated 'Fast Track' Rehabilitation Service for Multi-Trauma Patients: A Non-Randomized Clinical Trial in the Netherlands | PLOS ONE

A First Successful Pilot Project for CATALIS' New FAST TRACK Evaluation Service: 4 Quebec Institutions Ranked in the Top 5 of the World's Fastest Sites - CATALIS
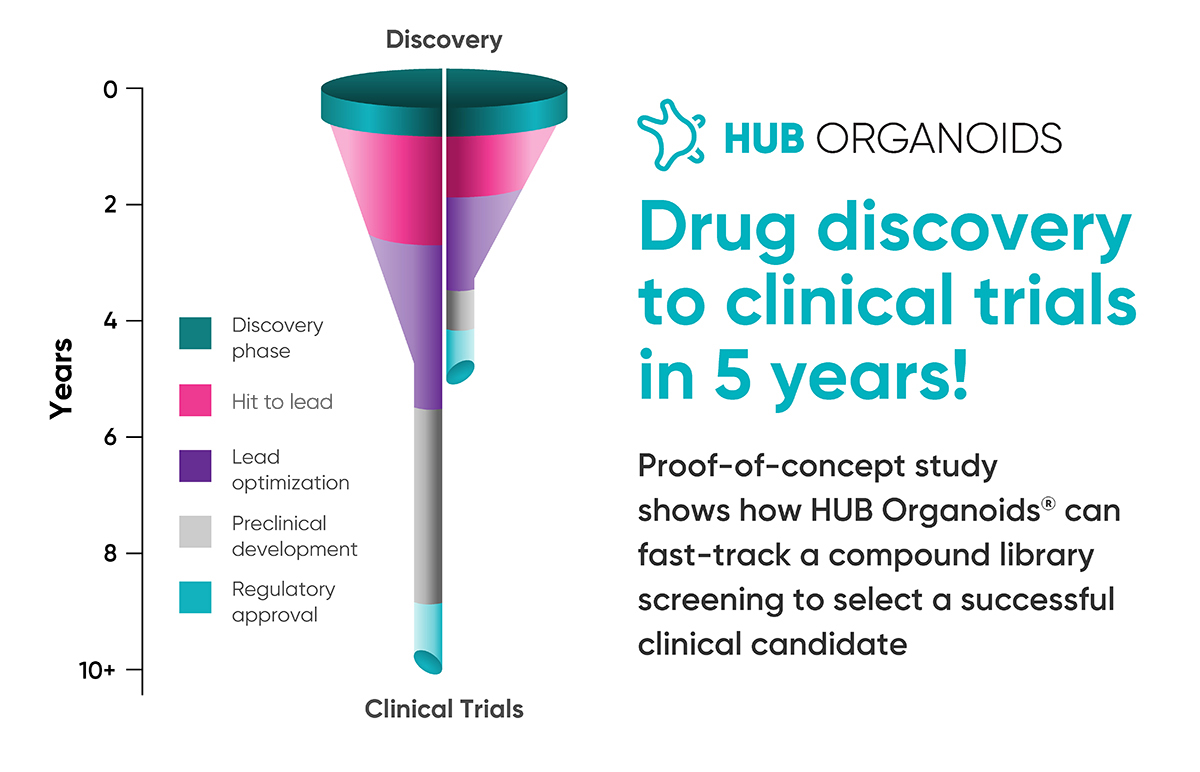
First clinical candidate developed using HUB Organoids makes it to the clinical trials within 5 years | HUB Organoids
FDA Moves Against Fast Track Vaccines - News about Energy Storage, Batteries, Climate Change and the Environment

Avantor Clinical Services supports the need to fast-track clinical trials related to COVID-19 disease progression | Avantor

Fast-track review of clinical trials for non-COVID 19 research to continue - Health Research Authority